
These hydrogen production pathways result in virtually zero greenhouse gas and criteria pollutant emissions however, the production cost needs to be decreased significantly to be competitive with more mature carbon-based pathways such as natural gas reforming. Hydrogen production via electrolysis is being pursued for renewable (wind, solar, hydro, geothermal) and nuclear energy options.

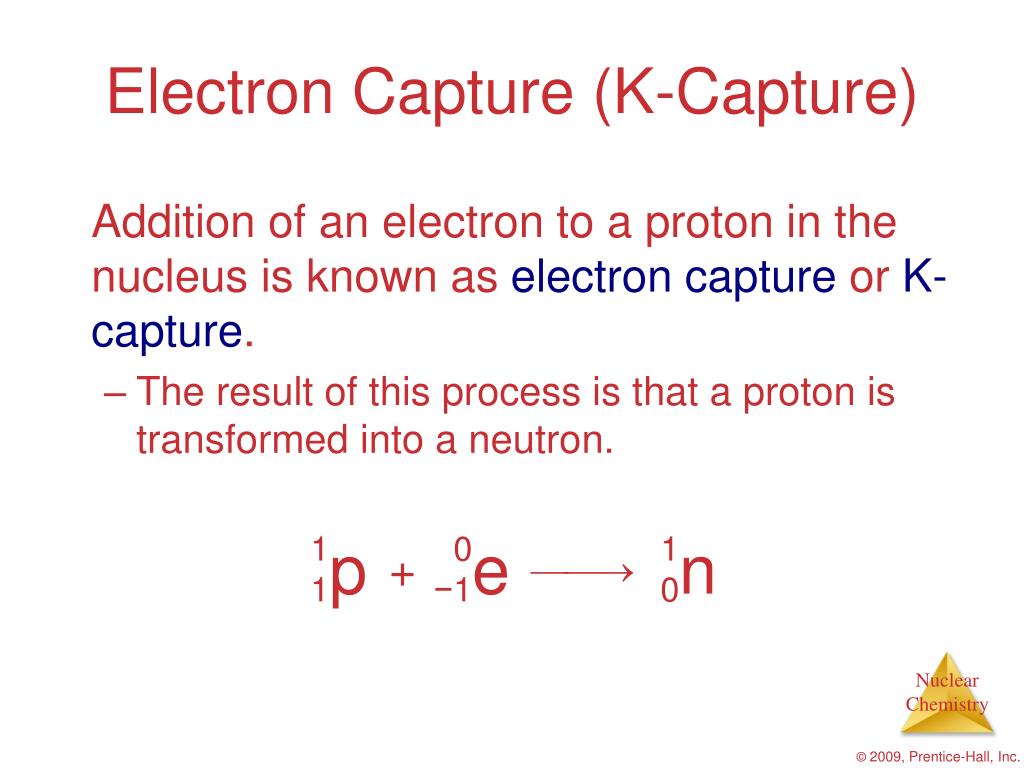
In many regions of the country, today's power grid is not ideal for providing the electricity required for electrolysis because of the greenhouse gases released and the amount of fuel required due to the low efficiency of the electricity generation process. The source of the required electricity-including its cost and efficiency, as well as emissions resulting from electricity generation-must be considered when evaluating the benefits and economic viability of hydrogen production via electrolysis. Hydrogen produced via electrolysis can result in zero greenhouse gas emissions, depending on the source of the electricity used. Why Is This Pathway Being Considered?Įlectrolysis is a leading hydrogen production pathway to achieve the Hydrogen Energy Earthshot goal of reducing the cost of clean hydrogen by 80% to $1 per 1 kilogram in 1 decade ("1 1 1"). The solid oxide electrolyzers can effectively use heat available at these elevated temperatures (from various sources, including nuclear energy) to decrease the amount of electrical energy needed to produce hydrogen from water. Advanced lab-scale solid oxide electrolyzers based on proton-conducting ceramic electrolytes are showing promise for lowering the operating temperature to 500°–600☌. Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700°–800☌, compared to PEM electrolyzers, which operate at 70°–90☌, and commercial alkaline electrolyzers, which typically operate at less than 100☌). The oxygen ions pass through the solid ceramic membrane and react at the anode to form oxygen gas and generate electrons for the external circuit.Steam at the cathode combines with electrons from the external circuit to form hydrogen gas and negatively charged oxygen ions.Solid oxide electrolyzers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O 2-) at elevated temperatures, generate hydrogen in a slightly different way. Newer approaches using solid alkaline exchange membranes (AEM) as the electrolyte are showing promise on the lab scale. Electrolyzers using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years.

Anode Reaction: 2H 2O → O 2 + 4H + + 4e - Cathode Reaction: 4H + + 4e - → 2H 2Īlkaline electrolyzers operate via transport of hydroxide ions (OH -) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side.
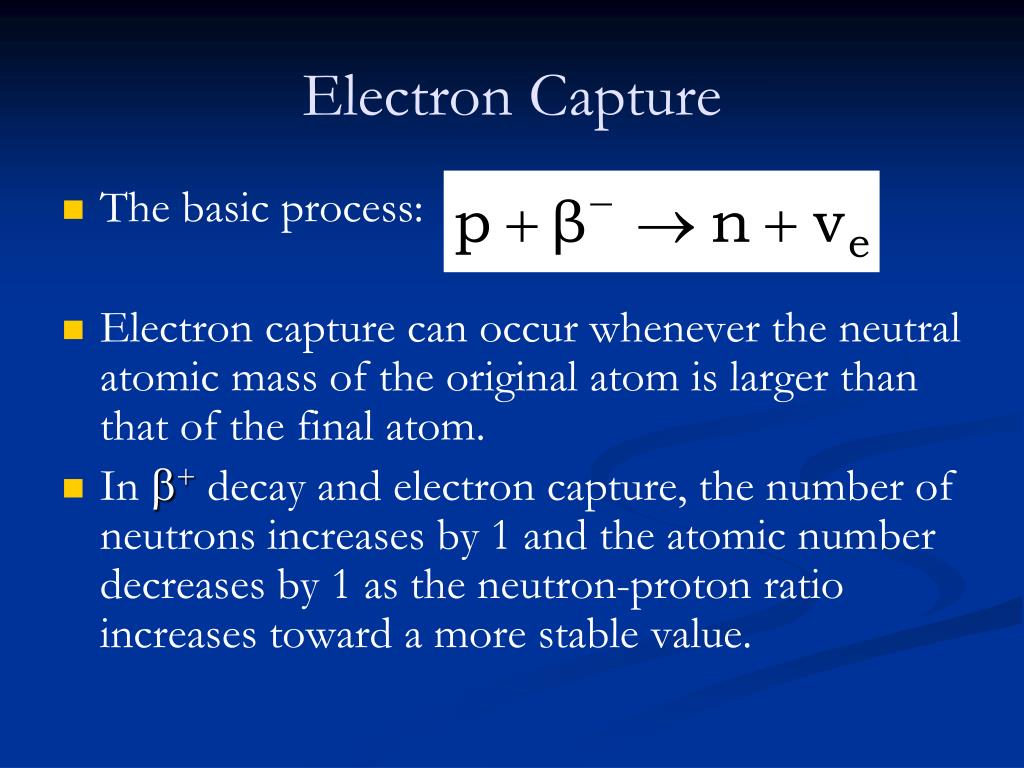
At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas.The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode.Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons).In a polymer electrolyte membrane (PEM) electrolyzer, the electrolyte is a solid specialty plastic material. Polymer Electrolyte Membrane Electrolyzers Different electrolyzers function in different ways, mainly due to the different type of electrolyte material involved and the ionic species it conducts. Like fuel cells, electrolyzers consist of an anode and a cathode separated by an electrolyte.


 0 kommentar(er)
0 kommentar(er)
